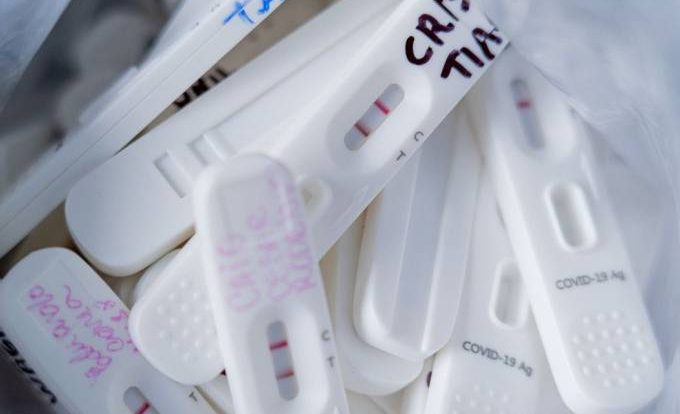
The National Health Surveillance Agency (Anvisa) has postponed the discussion to determine whether to allow the use of Automatic test for Covid-19 virus in Brazil. The decision was made at the college board meeting, by four votes to one, this Wednesday, 19. The deadline for a new analysis should be up to 15 days, during which time steps will be taken with the ministry. It is valid to request more information about creating a general policy for the use of tests.
last Thursday the 13th, The Ministry of Health asked the agency to approve the examination that can be performed at home.
This type of exam does not require professional supervision to perform and is an approved strategy in the United States and the United Kingdom. In its request, the ministry said that self-testing would be a complementary procedure for examining and isolating people infected with the new coronavirus.
Anvisa’s third board director, Christian Rose Jordan, explained that the technical memorandum sent by the department to the agency was analyzed and was not recognized as the formalization of public policy, a requirement to regulate the use of the product in the state. However, he assessed that the mechanism could be established due to the progress of the epidemic in the country.
“Given the urgent public health need, these regulations can be modified on an exceptional basis to identify and reduce transmission of a new ômicron variant.”
Christian addressed the concern about the correct implementation of the tests, to avoid false negative or false positive results, as well as with the registration of cases of the disease, given that Covid-19 is from Compulsory notification.
Director Romison Rodriguez Motta, of the Fourth Council, suggested an investigation and a resumption of discussion within a period of up to 15 days. The vote was followed by the two other directors, Alex Machado Campos and Meroz Souza Freitas, and the agency’s director general, Antonio Barra Torres.
“Approval along these lines, pure and only, will provide access to a screening tool that, in its wake, needs public policy,” said Anvisa Director and President, Antonio Barra Torres.
Among the questions that must be answered: how the data will be collected, the transformation of this data into a notification, where tests can be taken, the flow of positive patients, who will have to go to other places to complete the notification..

“Friendly zombie guru. Avid pop culture scholar. Freelance travel geek. Wannabe troublemaker. Coffee specialist.”