The world's first lung cancer vaccine, called BNT116, has begun trialling in the UK. The vaccine, produced by BioNTech, uses the same mRNA technology as the Covid-19 vaccine.
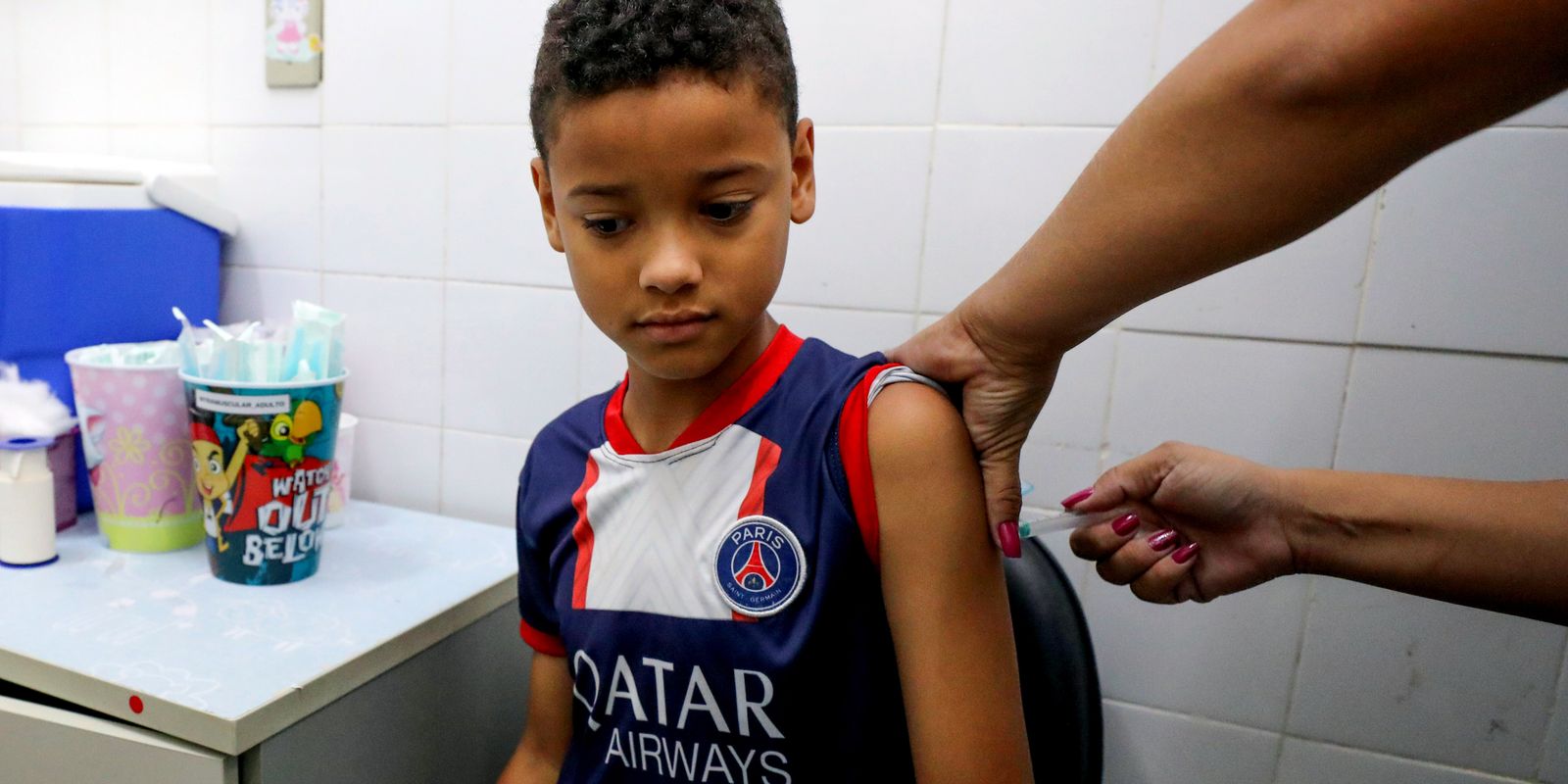
Janusz Racz, 67, was the first patient to participate in this phase of the study, receiving six consecutive injections of BNT116 at the National Institutes of Health Research Clinical Research Facility at the University of California, San Diego, last Tuesday (20).
Each injection contains genetic material that targets different parts of the tumor, with the goal of training 5 billion immune system cells.
Doctors say the treatment is more precise than chemotherapy, avoiding the side effects that affect healthy cells and can cause devastating effects. Rakez said the procedure was painless and less invasive than chemotherapy, a treatment he found difficult.
He expressed confidence that the new vaccine would not only help him, but others as well if it went into production quickly.
Around 130 patients with non-small cell lung cancer, the most common form of the disease, are willing to take part in the trial. Six hospitals in the UK are taking part in the process.
Lung cancer is the leading cause of cancer deaths worldwide, accounting for approximately 1.8 million deaths each year. Survival rates for patients with advanced forms of the disease, when tumors have spread, are particularly low.
The Phase 1 clinical trial, the first human study of BNT116, has been launched at 34 research centres in seven countries: the UK, the US, Germany, Hungary, Poland, Spain and Turkey. The vaccine teaches the body how to recognise and kill cancer cells, preventing them from returning.
mRNA works by providing the immune system with tumor markers, priming the body to fight cancer cells that express these markers. The goal is to boost the immune response to cancer while sparing healthy cells, unlike chemotherapy.
Siu Ming Lee, a consultant oncologist at University College London Hospitals NHS Foundation Trust, who is leading the trial in the UK, said the treatment is easy to administer and can target specific antigens on cancer cells, making it the next major stage in treating the disease.
Lee, who has researched lung cancer for 40 years, said that in the beginning, few people believed in the effectiveness of chemotherapy.
Currently, about 20% to 30% of stage IV patients survive on immunotherapy, and there is speculation that an mRNA vaccine could improve survival rates.
Lee hopes the vaccine will pass phases 2 and 3 of clinical trials and become the global standard of care, saving many patients.
Early tests of similar vaccines in other types of cancer have shown promising results, with reduced tumor size and risk of recurrence. This is the first time BioNTech’s vaccine has been tested in humans, and the trial will check for significant side effects.
Depending on the results, doctors intend to modify the vaccine to increase its clinical effectiveness.
Rakez, an AI scientist, said his profession motivated him to participate in the test, realizing that scientific progress, especially in medicine, depends on voluntary participation in research like this.

“Friendly zombie guru. Avid pop culture scholar. Freelance travel geek. Wannabe troublemaker. Coffee specialist.”