While looking into the eyes of his dog, Aretha, publicist Paolo Peregrino, 61, is thinking of going out for a walk with her around the apartment where he lives, in Niteroi (RJ).
Daily work is a breakthrough for him. Less than three months ago, Paolo was hospitalized with complications from hospital-acquired sepsis shortly after undergoing innovative cancer treatment.
In 2010, the publisher was diagnosed with prostate cancer, and eight years later, with non-Hodgkin lymphoma, underwent several chemotherapy sessions and even a bone marrow transplant, but to no avail.
“[Quando descobrimos] First prostate cancer, the doctor decided not to do anything because the tumor was encapsulated [localizado dentro de uma membrana]without the risk of spreading. After a year or two, we saw that there were two more [tumores]. And then when I went to start treatment, we noticed metastases in the legs already,” he said.
Since then, she has had several chemotherapy sessions and dozens of morphine shots for pain as well as a transplant. Because of the tumor, he even developed purpura, an autoimmune disease.
Until he received CAR T-cell treatment at the Hospital das Clínicas in São Paulo. Since then, he has had complete remission of the tumor.
“I feel great. Of course I still need to be examined, and see my exams periodically, but I don’t feel pain anymore, I sleep well, I eat well. I still feel tired and I’ve lost a lot of muscle, but little by little I’m getting back to doing my daily activities, Like walking here at home with my dog,” he said.
Paolo was included in a study conducted by the Center for Cellular Therapy at HC, in partnership with the USP Medical School of Ribeirao Preto and the Butantan Institute. In addition to the announced, 12 other patients have already been treated at Nutera (Advanced Treatment Centre). “The results are very promising. They are patients with very poor health and advanced stage of cancer,” said Vanderson Rocha, the physician leading the research.
CAR-T uses immune system cells (known as T lymphocytes) from the patient that are genetically modified to recognize and attack cancer cells.
The treatment has been successful in treating some leukemias, lymphomas, and leukemias, but there is no evidence of its effectiveness against solid tumours. In these cases, chemotherapy, radiotherapy, or treatments such as immunotherapy are most effective.
This technology has been approved by Anvisa (National Health Surveillance Agency) since 2021 for what is called compassionate use in Brazil. This practice is adopted when no other approach is possible and treatments that are still being studied are used to try to save the patient’s life.
In addition, in July 2022 the agency approved the first national development clinical trial of CAR-T cells for cancer treatment at Israelita Albert Einstein Hospital.
All patients included in the study are users of SUS (Sistema Único de Saúde), and the research has received financial support from the Ministry of Health.
“We have the only CAR-T research program in the country, which will also help cancer patients at the Municipal Vila Santa Catarina Hospital. We have developed our own product and hope to get Anvisa approval for its use soon. [na população]said the director of research at Einstein, Luiz Vicente Rizzo.
In general, patients with highly aggressive cancers, or those with compromised immune systems, have more difficulty generating their own immune response, which is why CAR-T therapy can be an important ally.
One of them is municipal maid Ana Claire Marquis Diogenes, 61, who was first diagnosed with non-Hodgkin lymphoma in 2017. After treatment with chemotherapy in her hometown of Fortaleza, Ceará, she went through a period of remission until 2021 when her first relapse came.
Like Paolo, Ana underwent a bone marrow transplant in January 2021. The process is similar to CAR-T therapy, but it’s bone marrow cells, while CAR-T therapy uses lymphocytes. Even after implantation, a new server iteration occurred in 2022.
This is when my first doctor suggested I contact a doctor in São Paulo and was invited to participate in the study on Einstein. I was hospitalized on March 1st. [de 2023] And on day 11, we actually infused CAR-T cells,” she said, and, as I mentioned, after 60 days of treatment, she already had complete remission of the cancer.
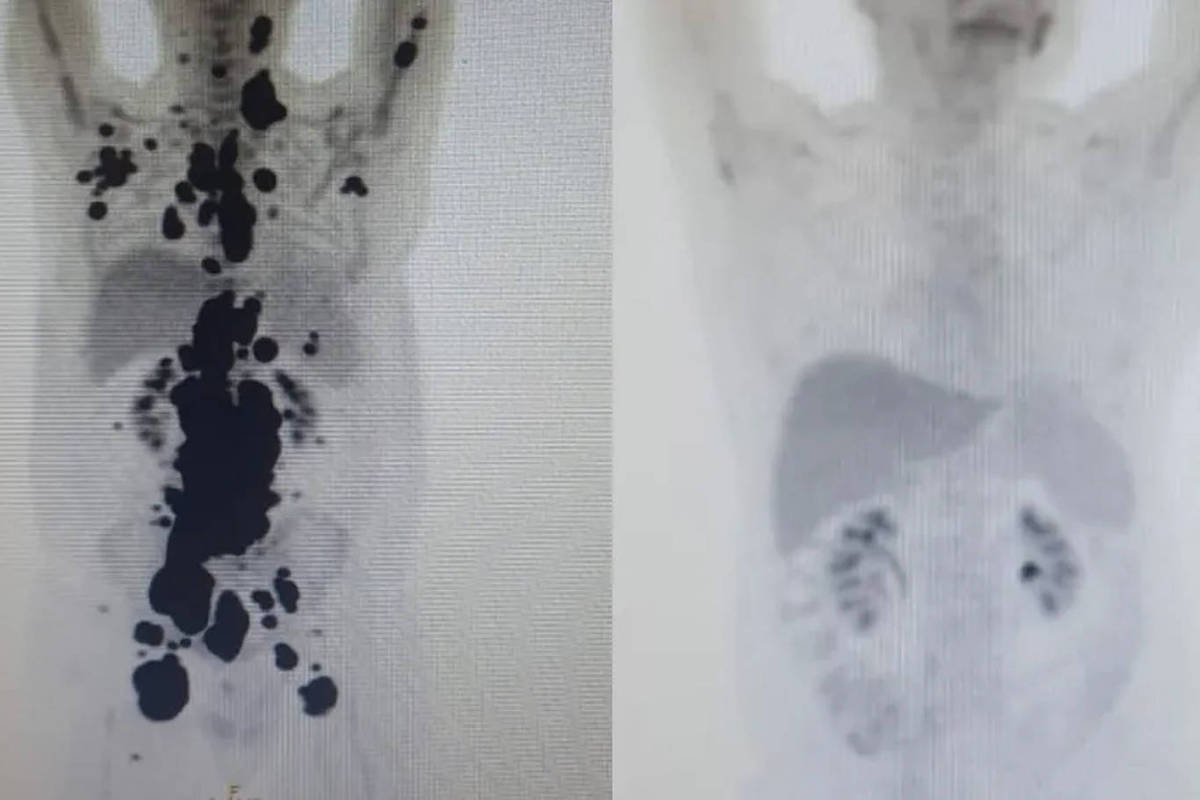
The patients are special cases of aggressive tumors, which have not been effective in conventional therapy, and which may be included in the studies. PET scans (computerized tomography) already indicate remission of the tumor in both cases, but it is still too early to talk about a cure.
This is because remission occurs when there is no more focus on the tumor in the body, whereas treatment, according to international clinical protocol, is when there is no more focus on the tumor and remission remains after five years of treatment.
To date, no patients have been included in the study with a follow-up of longer than 1 year and 4 months. Therefore, no treatment case has been described so far.
However, Ana and Paolo are already breathing a sigh of relief. “It’s a very big hope because there are many people doing research every day so that this treatment, which is less invasive, gets to more people,” El-Khadem said.
Paolo considers his participation in the study can serve as an example. “To everyone who looked after me in the hospital, I said that you were an essential part of my victory. I hope that others in the future will not have to go through the same difficulty as I did.”